510(k) Cleared for Treating In-Stent Restenosis with Illuminating Data
Pantheris has received 510(k) clearance for treating in-stent restenosis (ISR), expanding the Lumivascular atherectomy platform into new clinical applications. The only PAD treatment with onboard image guidance, Pantheris optimizes ISR therapy by combining intravascular visualization with a targeted mechanism of action for safe and effective outcomes.
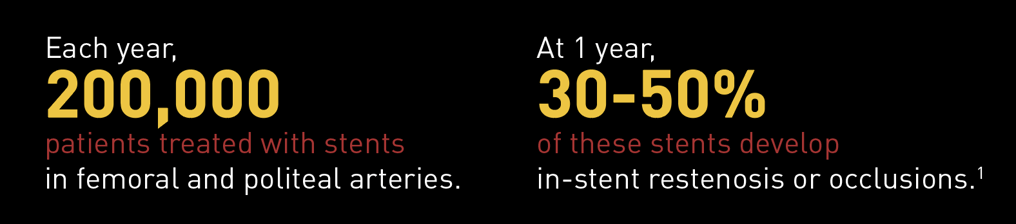
The Problem: In-Stent Restenosis
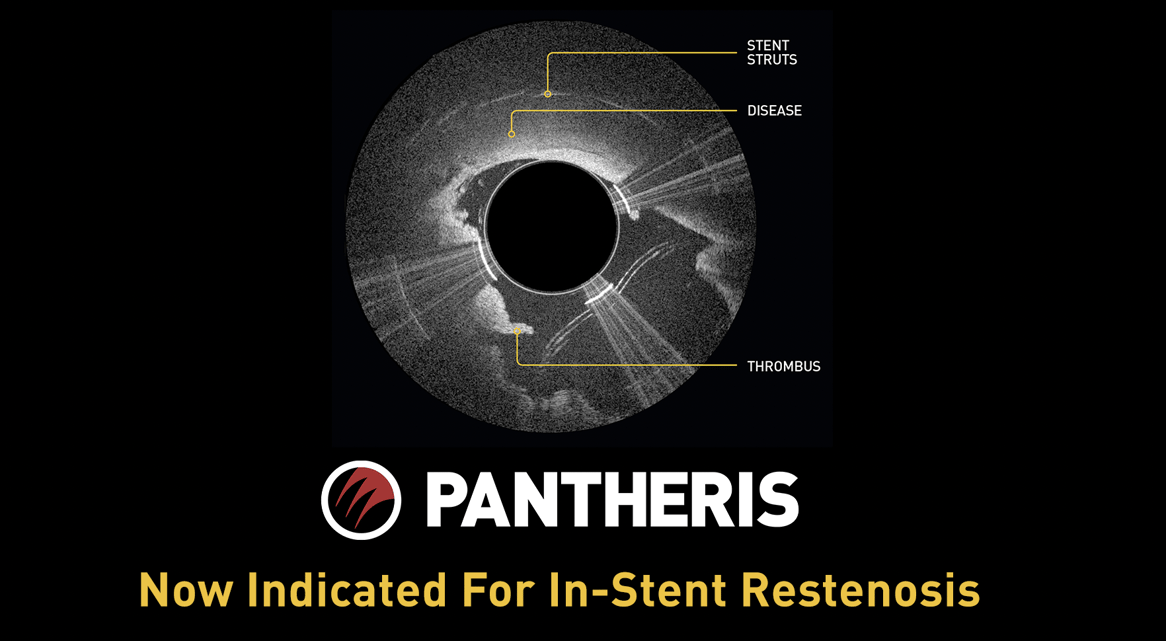
The Solution: Pantheris
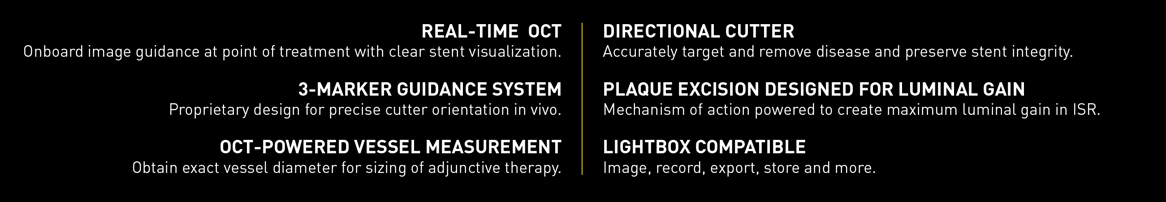
Real-Time OCT Imaging

The Data: INSIGHT ISR Study
97 PATIENTS* I 17 SITES
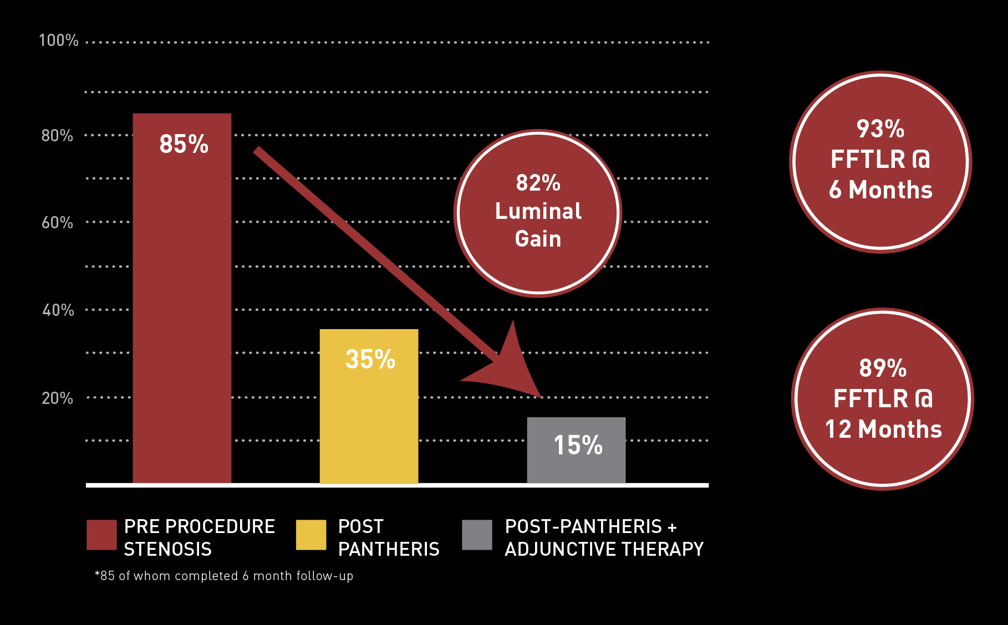
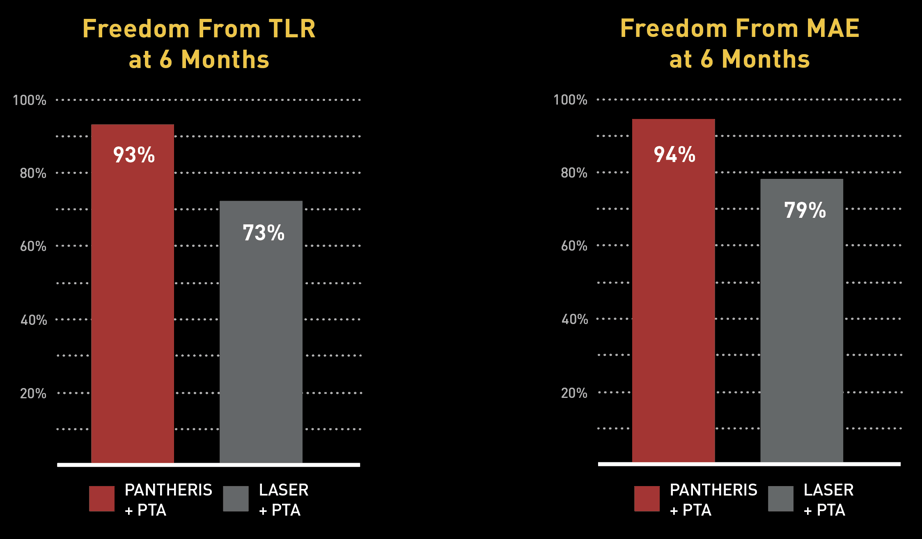
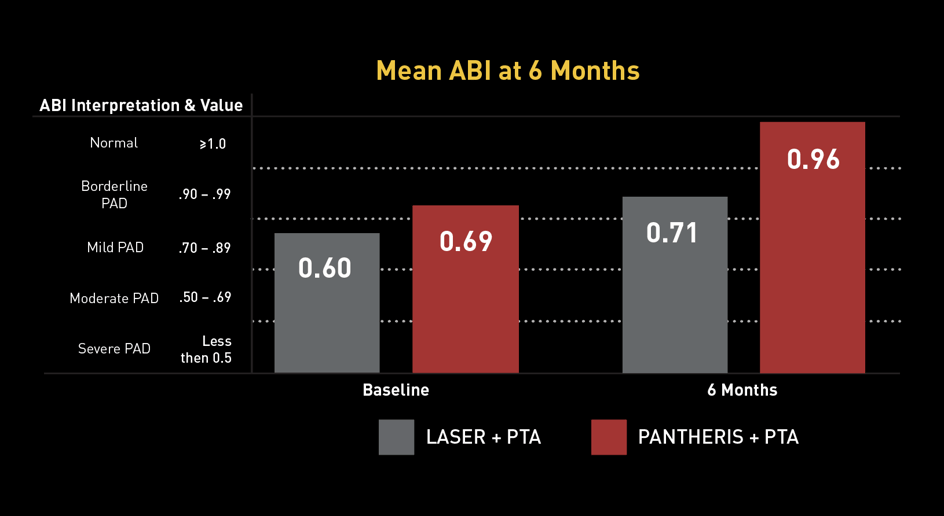

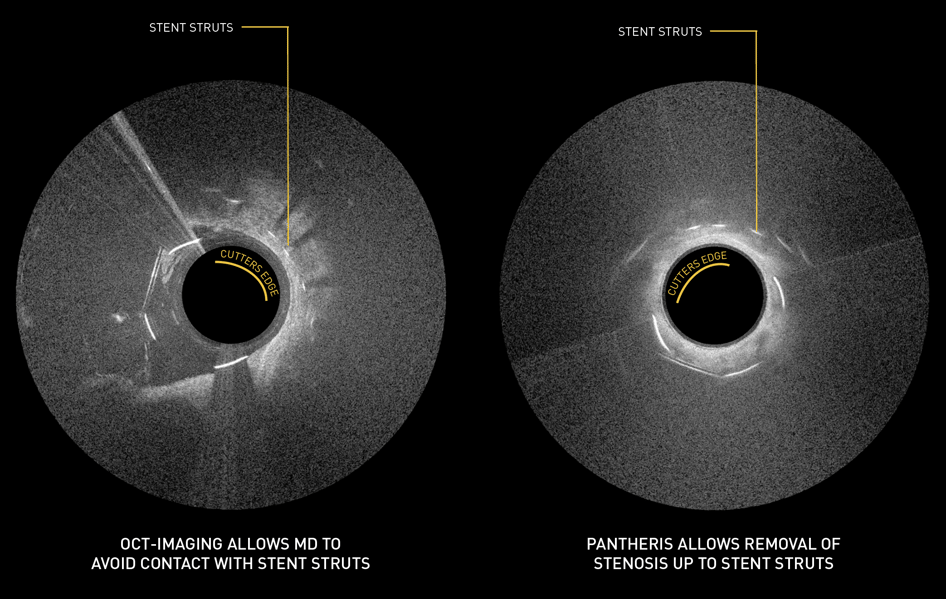
Principal Investigator Dr. Glen Schwartzberg: "The Pantheris system’s combination of onboard image-guidance and a directional excision mechanism provides significant clinical advantages in treating in-stent restenosis not available with any other therapy,” noted Dr. Schwartzberg. “This technology allows the operator to target only the blockage and maximize the channel for restored blood flow while avoiding negative interactions with clearly delineated stent struts. Based on the results of the INSIGHT study and my personal experience with the device, I believe that Pantheris can help physicians safely and effectively treat many ISR patients that previously may not have had any other options available and reduce the need for repeat interventions and more invasive surgeries."
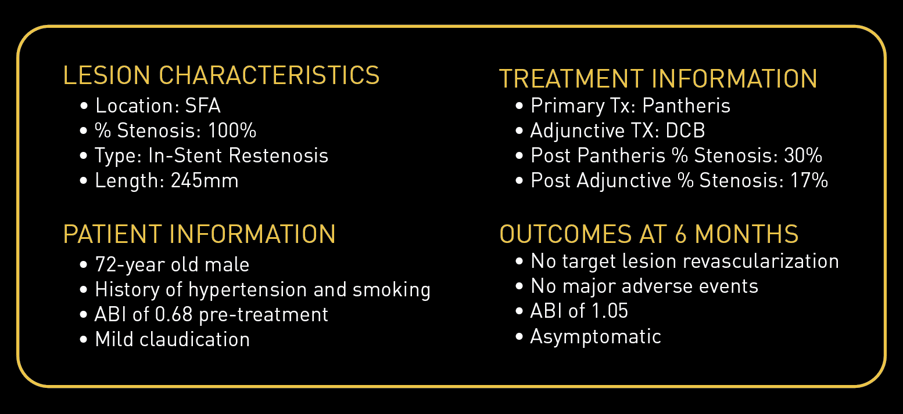
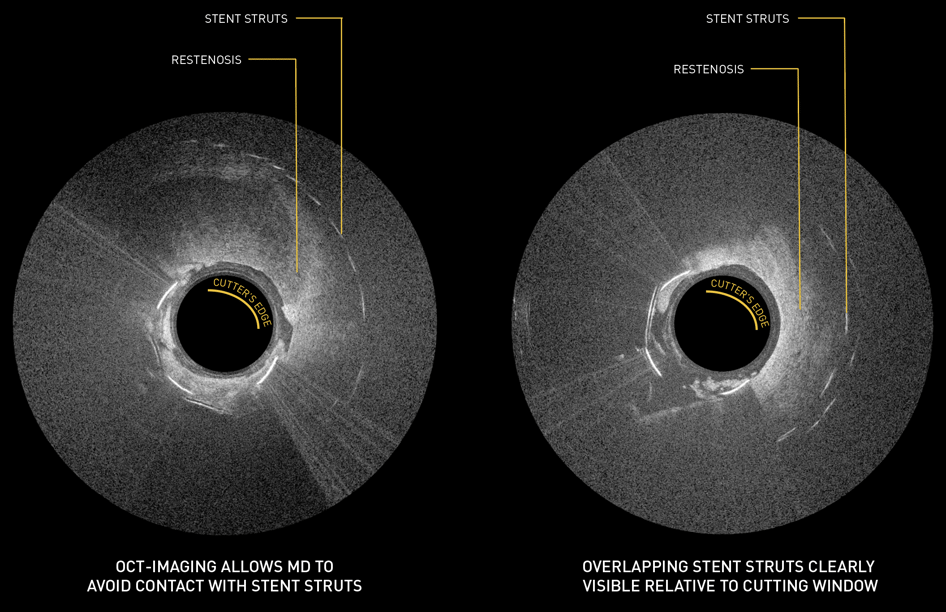
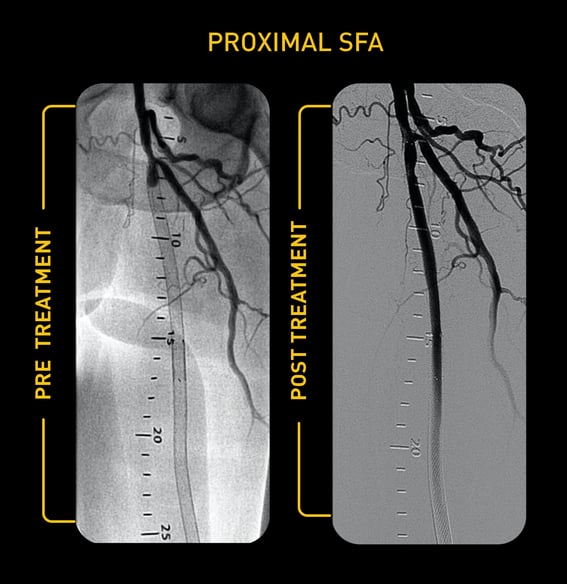
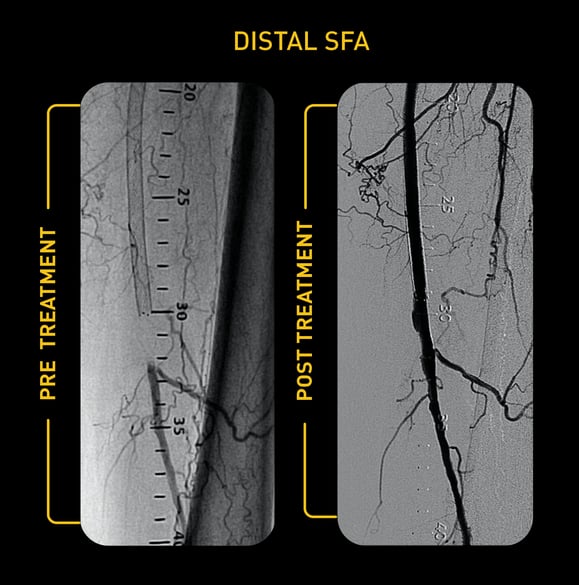
Case Study: Pantheris Treating ISR
Courtesy of Gary Fishbein, MD
OCT Snapshots
VISUALIZATION. PRECISION. SAFETY.
References:
- Dippel, E. TCT, 2014. Laird J R. Limitations of Percutaneous Transluminal Angioplasty and Stenting for the Treatment of Disease of The Superficial Luminal and Popliteal Arteries. J Endovase Thur. 2006; 13 02: II3D - II4D.
- All INSIGHT Data on file at Avinger.
- All laser + PTA from "Dippel et al. Acc: Cardiovascular Interventions 2015.